We are pleased to recommend another webinar:
Analytical design space modeling in accordance with ICH Q12 and proposed Q14: Using a novel approach for in silico method development and robustness assessment
30. Juni 2020 – 16:00 BST / 17:00 CEST / 08:00 PDT / 11:00 EDT
For the registration please click on the following link.
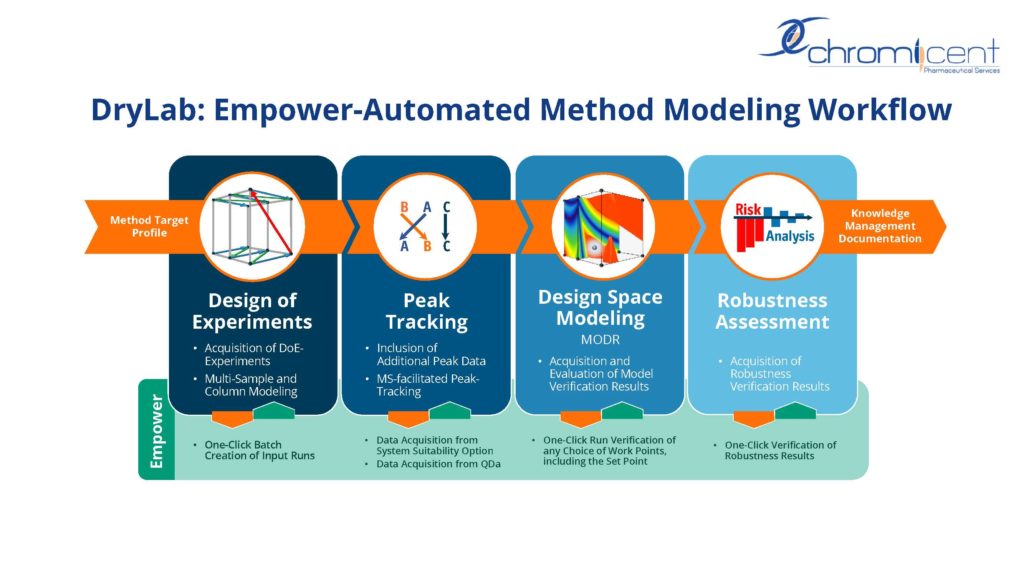
The successful implementation of Analytical Quality by Design (AQbD) workflows across analytical development has become strategically relevant to the pharmaceutical industry. Scientific design space modeling has proven effective in yielding high levels of robustness, critical to successful transfer and routine use of analytical methods across laboratories. AQbD approaches meet ICH guidance on scientific and risk-based analytical procedure development as suggested in Q12 and proposed Q14, including effective regulatory communication and providing the basis for post-approval flexibility.
In this upcoming webinar, hear from Dr. Pankaj Aggarwal, Principal Scientist at Pfizer, as well as Dr. Alexander H. Schmidt and Mijo Stanic, co-founders of Chromicent GmbH, on how automated AQbD modeling can be used as a strategic approach across analytical operations of a large pharmaceutical company and how system-wide implementation using two established software packages for method modeling can allow for seamless automation of the AQbD modeling workflow.
Watch this webinar to learn:
- How to use an automated AQbD approach to achieve consistent performance across the method lifecycle
- How to implement an automated robustness assessment
- How to efficiently report analytical development outcomes
- How to improve laboratory efficiency using time-saving approaches that address complex, labor-intensive method work
- Strategies to obtain post-approval flexibility
A look behind the scenes – Dr. Alexander H. Schmidt and Mijo Stanic recording the webinar

Dr. Alexander H. Schmidt 
Mijo Stanic
