- Pills & Pipettesby ChromicentTo celebrate Chromicent’s anniversary, the entire team visited the Museum of Technology and were guided through the history of Schering AG in Berlin with the… Read More »Pills & Pipettes
- 10th anniversary of Chromicentby ChromicentDear friends and customers of Chromicent, At Chromicent, we usually look ahead – but this spring we are taking a look back: at 10 years… Read More »10th anniversary of Chromicent
- Publication: ICH Q14-inspired novel approachby ChromicentChromicent’s latest publication is a case study inspired by the ICH Q14 guideline on method development under analytical quality by design (AqbD) principles using the… Read More »Publication: ICH Q14-inspired novel approach
- ICH Q14 –Development of analytical methodsby ChromicentChromicent is pleased to invite you to the following event again this year: The Chromicent is offering its seminar and laboratory facilities for the event… Read More »ICH Q14 –Development of analytical methods
- LIMSby ChromicentOptimising laboratory processes while maintaining data integrity and complying with legal regulations is one of the key challenges for GLP-certified pharmaceutical service providers such as… Read More »LIMS
- News from the Chromicentby ChromicentDear customers and friends of Chromicent, Autumn at Chromicent is once again full of news. You can find the current Chromicent news in our information… Read More »News from the Chromicent
- Laboratory equipmentby ChromicentChromicent is now able to use an ACQUITY Premier System from Waters. The ACQUITY Premier System is a consequent advancement of the previous UPLC systems.… Read More »Laboratory equipment
- Alliance iS Seminar in Berlinby ChromicentIntuitive Simplicity – with your new indispensable ally in the lab. We look forward to presenting the Alliance iS HPLC System by and with Waters… Read More »Alliance iS Seminar in Berlin
- Biopharma User Meeting (Waters)by ChromicentThe established and popular Waters Biopharma User Meetings will take place again this year as live events. Experts from industry and academia provide practice-oriented information… Read More »Biopharma User Meeting (Waters)
- BSFZ Seal for Innovation in R&Dby ChromicentChromicent is a pharmaceutical service provider that develops chromatographic methods on behalf of its customers. In addition, we conduct innovative and proactive research on drug… Read More »BSFZ Seal for Innovation in R&D
- SFC Europe 2023by ChromicentSFC Europe 2023, organised by the Green Chemistry Group, took place from 14-16 May 2023 at the Novartis Pavilion in Basel, Switzerland. Chromicent was involved… Read More »SFC Europe 2023
- European AutoPurification Meetingby ChromicentAt the end of April, Waters GmbH, in close and proven successful cooperation with Chromicent, invited participants to the two-day European AutoPurification Meeting. Sixty interested… Read More »European AutoPurification Meeting
- GMP Certificateby ChromicentChromicent’s GMP compliance confirmation has been issued for a further three years by the local drug authortiy in accordance with§ 64 of the German Medicinal… Read More »GMP Certificate
- Purification & Automation Meetingby ChromicentDate: 26. April 2023 – 27. April 2023 Time: 11-17:30h / 9-16h Location: Berlin, Chromicent Language: English Organised by: Waters GmbH For more information and… Read More »Purification & Automation Meeting
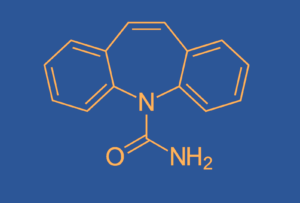
- Nitrosamine in sitagliptin medicinesby ChromicentContamination of medicines by nitrosamines is an issue that is constantly gaining in importance. Currently, the pharmaceutical giant Merck is confronted with nitrosamine impurities in… Read More »Nitrosamine in sitagliptin medicines
- News from the Chromicentby ChromicentDear customers and friends of Chromicent, we wish you a successful start into the new year! Chromicent is pleased to be able to combine its… Read More »News from the Chromicent
- Merry Christmasby ChromicentDear customers and friends of Chromicent, the Chromicent team wishes you a Merry Christmas, a peaceful holiday season and a Happy New Year.
- Multidimensional Chromatographyby ChromicentMultidimensional chromatography is now part of Chromicent’s portfolio. The limitation of resolution in terms of peak purity and peak capacity is one of the greatest… Read More »Multidimensional Chromatography
- Avantor Chrom Forum – September 2022by ChromicentAvantor’s information forums on the topic of chromatography look back on a long tradition – and at the same time are always close to the… Read More »Avantor Chrom Forum – September 2022
- Sustainability in analytics – SFCby ChromicentWhen we talk about method development at Chromicent – we always talk about sustainability. Chromicent’s Method LifeCycle Management (MCLM) is exactly that: sustainable. This means… Read More »Sustainability in analytics – SFC
- GC – gas chromatographyby ChromicentChromicent is pleased to announce an expansion of its portfolio and capacity in the field of gas chromatography. The new GC system will be operated… Read More »GC – gas chromatography
- The Chromicent at a glanceby ChromicentDear customers and friends of Chromicent, the comprehensive Chromicent portfolio at a glance. Of course also available as PDF for download:
- News from the Chromicentby ChromicentDear customers and friends of Chromicent, We hope you have had a good start to 2022. At Chromicent, the start of the new year is… Read More »News from the Chromicent
- Nitrosamine – New publicationby ChromicentNitrosamines continue to be an important topic in drug safety. Chromicent conducts intensive research in this area and thus makes its contribution to drug suply… Read More »Nitrosamine – New publication
- European Extractables and Leachables Forumby ChromicentThe migration of potential harmful substances from packaging materials is one of the central issues in health protection. This concerns manufacturers of pharmaceutical products as… Read More »European Extractables and Leachables Forum
- Merry Christmasby ChromicentDear customers and friends of Chromicent, the Chromicent team wishes you a Merry Christmas, a peaceful holiday season and a Happy New Year.
- Nitrosamine Webinarby ChromicentWaters GmbH is hosting a webinar on the topic: „Genotoxic Impurities: Nitrosamines LC-MS TOF screening analysis with automated sample preparation“. Save the Date: 7. Dezember… Read More »Nitrosamine Webinar
- News from the Chromicentby ChromicentDear customers and friends of Chromicent, we have good news for you again. You can find our detailed information letter here as a PDF:
- Detection methods for LC and SFC at the Chromicentby ChromicentChromicent, the special laboratory for chromatographic methods in the pharmaceutical field, offers a comprehensive range of detection methods for LC and SFC. We are continuously… Read More »Detection methods for LC and SFC at the Chromicent
- Autumn of detectorsby ChromicentAs soon as the first leaves turn colourful, autumn is all about detectors for Chromicent. With the renewed expansion of our portfolio, we can now… Read More »Autumn of detectors
- Spring Awakening – Events & Webinarsby ChromicentThe month of May is all about events & webinars at Chromicent. We are pleased about the growing interest of our customers in lectures on… Read More »Spring Awakening – Events & Webinars
- Stability Testingby ChromicentStability tests according to ICH Q1A are an established part of Chromicent’s service portfolio. In April, we adapted and expanded the existing range of services… Read More »Stability Testing
- 7 years Chromicentby Chromicent7 years of Chromicent – and we say a warm thank you to all our colleagues and companions, customers and friends, and especially to our… Read More »7 years Chromicent
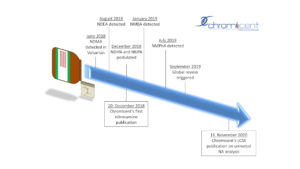
- New publication: Nitrosaminesby ChromicentThe first detection of nitrosamines in valsartan preparations took place two years ago. Since then, it has become obvious that the problem of contamination of… Read More »New publication: Nitrosamines
- BioAccord – Nitrosamines and moreby ChromicentIn response to the increasing demand for large molecule analyses, we at Chromicent are currently testing the BioAccord system from Waters. The background and further… Read More »BioAccord – Nitrosamines and more
- Ion Chromatography & Eventsby ChromicentDear customers and friends of Chromicent, we would like to start by wishing you an happy new year. As you have come to expect from… Read More »Ion Chromatography & Events
- Merry Christmasby ChromicentDear customers and friends of Chromicent, The Chromicent team wishes you a Merry Christmas, a peaceful holiday season and a Happy New Year.
- News from the Chromicentby ChromicentDear customers and friends of Chromicent, our information letter for autumn 2020 has been released. You will find it in the following for download as… Read More »News from the Chromicent
- Take the next step with usby ChromicentThe Chromicent is now using a BioAccord system from Waters. What is special about it? BioAccord is a Ready to Use LC-MS system that enables… Read More »Take the next step with us
- Digitization – Key Topic of our Timeby ChromicentSeptember at Chromicent was all about digitalization. In cooperation with the HTW Berlin, we have subjected Chromicent to a digital checkup as part of the… Read More »Digitization – Key Topic of our Time
- EMA – Statement Nitrosamineby ChromicentThe EMA has published a final statement on nitrosamine impurities in medicinal products. The full report can be found *here*. The publication of Chromicent is… Read More »EMA – Statement Nitrosamine
- News from the Chromicentby ChromicentDear customers and friends of Chromicent, we have good news and would like to share it with you. You will find our detailed information letter… Read More »News from the Chromicent
- New: Size Exclusion Chromatography (SEC)by ChromicentChromicent has enhanced its portfolio with size exchange chromatography (SEC). This chromatographic method enables us to separate molecules in solution by size (hydrodynamic volume) and… Read More »New: Size Exclusion Chromatography (SEC)
- ICH Q2/Q14 Analytical Procedure Life Cycle Managementby ChromicentThis conference provides a comprehensive overview of the new ICH Quality Guideline Q14 on Analytical Procedure Development, and the revised Q2 Guideline on Validation of… Read More »ICH Q2/Q14 Analytical Procedure Life Cycle Management
- Analytical Design Space Modelingby ChromicentWe are pleased to recommend another webinar: Analytical design space modeling in accordance with ICH Q12 and proposed Q14: Using a novel approach for in… Read More »Analytical Design Space Modeling
- Challenge: Nitrosaminesby ChromicentIn the field of pharmaceutical safety, nitrosamines remain a crucial issue. In a webinar entitled “Analysis of nitrosamines in APIs by LC/MS: How to face… Read More »Challenge: Nitrosamines
- Method LifeCycle Management from Chromicent GmbH speeds up drug development considerably. Contribution of the Berlin-based company to coping with the “Corona crisis”by ChromicentEvents such as the coronavirus pandemic make us aware that access to innovative, affordable and safe medicines is one of the challenges of our time… Read More »Method LifeCycle Management from Chromicent GmbH speeds up drug development considerably. Contribution of the Berlin-based company to coping with the “Corona crisis”
- The current situationby ChromicentDear customers and friends of Chromicent, the Chromicent, like all of us, faces great challenges. Our SOP “Pandemic Plan” was activated at the beginning of… Read More »The current situation
- Nitrosamineby ChromicentIn accordance with Article 31 of Directive 2001/83/EC, the EMEA expects all pharmaceutical manufacturers to provide information on nitrosamines contained in medicinal drugs by the… Read More »Nitrosamine
- Nitrosamine – SFC capacity expandedby ChromicentDear customers and friends of Chromicent, as you can see in our last year news, nitrosamines in active ingredients are still a hot topic !… Read More »Nitrosamine – SFC capacity expanded
- Food and Environmental Forum 2020by ChromicentChromicent is pleased to present our method for the detection of pyrrolizidine alkaloids by LC-MS/MS and SFC-MS/MS developed by our project manager Maike Rehn at… Read More »Food and Environmental Forum 2020
- The Chromicent wishes you a happy new year!by ChromicentDear customers and friends of Chromicent, we wish you a successful new year and start with good news: At the end of 2019, the local… Read More »The Chromicent wishes you a happy new year!
- Merry Christmas and a successful 2020!by ChromicentDear customers and friends of Chromicent, we look back with pleasure on a successful year and would like to take this opportunity to thank you… Read More »Merry Christmas and a successful 2020!
- QbD-Lecture at the University of Paviaby ChromicentThe University of Pavia near Milan is one of the oldest, most prestigious and, if we may say, most beautiful European universities. Their history dates… Read More »QbD-Lecture at the University of Pavia
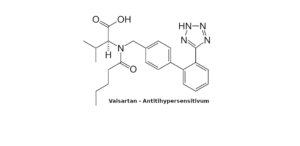
- Valsartan – new analysis method with SFCby ChromicentOur SFC specialist Sebastian Schmidtsdorff has developed a new method for testing sartans for contamination by nitrosamines. Our analysis using SFC-MS / MS is now… Read More »Valsartan – new analysis method with SFC
mail: info@chromicent.de tel: +49(0)30233289300